Streamlining Scientific Publishing: Best Practices for Sponsors and CROs
Medical writing is a fundamental driver of innovation and excellence in clinical research. With the clinical development landscape and publication practices continuously evolving to meet current needs, both contract research organizations (CROs) and sponsors need to be agile and innovative in their approach to tackling challenges that could hinder publication projects. Whatever the challenge, whether it be new trends, regulations, or guidelines; remote work; or staff changes, the dynamic between a sponsor and CRO remains critical to not only a clinical trial’s success but also any associated projects. For nearly two decades, TFS HealthScience has upheld ethical standards, precision, and scientific rigor to protect patients and provide a much-needed bridge between medical breakthroughs and clear, accessible scientific communications that foster trust and facilitate medical decision-making.
In this article, we explore strategies and best practices that TFS’s medical writers have found helpful in optimizing our interactions with sponsors and CROs to ensure smoother collaborations for streamlined communication of clinical trial results.
Essential Elements for a High-impact Scientific Publication
To better understand how CROs and sponsors can more effectively work together on publication projects, a better understanding of what they are trying to achieve is needed. A good scientific publication should be comprehensive, clear, accurate, and provide significant value to the target audience (typically the scientific or medical community but also patients). Together, medical writers and sponsors (or CROs) need to agree on quite a few key elements to ensure a high-quality, impactful publication.
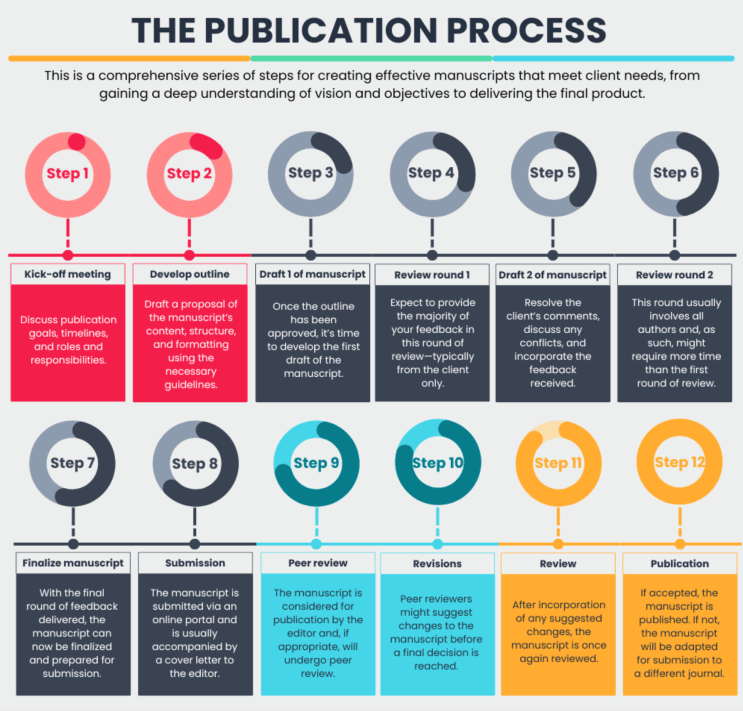
At the top of this list is the publication goal(s). Sponsors should consider who they are trying to reach and what they would like their target audience(s) to take away from the publication. Understanding these basic requirements goes a long way in streamlining manuscript development (see Fig. 1), which includes choosing the most appropriate journal to publish your results in. Things like impact factor, open access, altmetrics, and, of course, the aims and scope of the journal are fundamental in effectively reaching your target audience. Although medical writers are well aware of these basic considerations, their clients might not be.
It is on both the medical writer and sponsor to set the tone for a productive collaboration with open, honest, and frequent communication where important considerations, best practices, and expectations are discussed in detail and in a timely manner and embraced by both parties before the publication project commences.
Figure 1: A comprehensive series of steps for creating effective manuscripts that meet sponsors and CROs needs.
Common Roadblocks Encountered Along the Way for Sponsors and CROs
From the perspective of a CRO, the ideal sponsor or client is friendly, engaged, responsive, respectful, communicative, and open to trying new—more efficient and productive—ways of working. Experience tells us that this is also true for sponsors’ expectations of CROs. As a service provider, CROs are naturally also expected to deliver high-quality work as efficiently as possible. But things don’t always go precisely according to plan. Roadblocks along the publication process may occur and could be the result of a number of things:
- Poor communication and ineffective feedback loops: Poor communication can damage relationships and hamper the management and coordination of a project, especially if multiple teams, time zones, and workflows are involved. Not having robust feedback mechanisms in place for promptly addressing any issues that might arise could have the same effect.
- Misalignment of goals and expectations: The CRO and sponsor may have different expectations or priorities regarding the timeline, content, and messaging of the publication. Aligning these goals early on is essential to avoid delays later.
- Insufficient access or sharing of critical data or other study information: It is crucial for the medical writer(s) to have access to all the necessary data, including complete and accurate data. Confidentiality concerns might hinder access.
- Regulatory and compliance requirements: Adhering to the different regulatory guidelines for publication, such as Good Publication Practice (GPP) or the International Committee of Medical Journal Editors (ICMJE) standards, is a must. Sponsors might be unfamiliar with these or interpret them differently.
- Multiple review cycles with multiple parties: Manuscripts almost always require review from different stakeholders, including the sponsor and various study investigators. This aspect of manuscript development can make it difficult to collect feedback in a timely manner or keep everyone on the same page about the content or publication goals.
- Cultural or language barriers: If the CRO, the sponsor, and any other parties involved operate in different regions, language, and cultural differences can complicate communication, especially in interpreting regulatory guidelines or specific publication requirements.
Fostering Genuine Partnership with your CRO
Effective communication, early alignment, and clear documentation can help mitigate many of the aforementioned challenges. Fostering a meaningful partnership with your CRO will not only ease the current publication project but also streamline any future work. Here are some of the most important ways in which sponsors can enhance their collaboration with CROs and vice versa:
| Clear communication that fosters transparency and trust | Establish clear lines of communication from the outset. Sponsors should articulate their goals, expectations, and timelines to the CRO or medical writer(s) to ensure that everyone is aligned. Medical writers should highlight their experience in scientific publishing to their clients, reassuring them of their capabilities and expertise in any specific area. Both parties should strive to foster an open environment where everyone feels comfortable discussing challenges or asking questions. Full transparency builds mutual trust and helps resolve issues early on. |
| Collaborative planning, aligned objectives, and define roles and responsibilities | Work together to develop a realistic timeline that accounts for multiple drafts, review, and any potential delays. Here, again, medical writers typically have copious experience to guide the sponsor in the best way. Sponsors should be open to any relevant suggestions in this area. Early planning can help avoid last-minute rushes and ensure all parties have adequate time to perform their tasks. Along with an appropriate timeline comes a clear outline of who is involved in the project and their roles and responsibilities. Oftentimes review of a manuscript is required by other internal sponsor departments or external collaborators but are not mentioned during the planning stage. Inform all reviewers, especially busy clinicians, of the timeline and the overall publication process. This helps avoid misunderstandings, ensure accountability, and keep the project timeline on track. Discuss, in detail, the primary goals of the publication, including the target audience, key messages, and desired impact. Though aligned objectives may shift during manuscript development, having a clear line of communication will ensure that the final product, nevertheless, meets the expectations of all the stakeholders. |
| Comprehensive information and full awareness of regulatory guidelines and ethical standards | Sponsors should share all relevant data, background information, and study insights with the medical writer(s). This will provide the medical writer(s) with a good understanding of the study and facilitate the creation of high-quality, accurate, and engaging publications. Ensuring that all parties are aware of and adhere to the relevant regulatory guidelines and ethical standards for scientific publishing will ensure that the publication is also compliant. Allow the medical writer(s) to navigate these guidelines with you. Last but not least, medical writers should ensure their clients, if any doubt arises, of the safeguards in place to protect the confidentiality of their data—this is top priority for all CROs. |
| Timely feedback and review supported by the use of appropriate digital tools | Sponsors and any other stakeholders should review drafts promptly and provide constructive feedback. Tracking progress, managing deadlines, and communicating with project members don’t have to be labor intensive. Several digital communication tools and channels, such as Microsoft’s 365 cloud-based apps SharePoint, OneDrive, and Teams, are standard in our industry and have proven exceptionally useful in facilitating these activities. Similarly, co-authoring or collaborative authoring of manuscripts is powered by cloud-based apps (these days also integrated with artificial intelligence capabilities) to improve productivity and efficiency, supporting the quick turnaround of high-quality manuscripts. An open mind when it comes to using new technology is ideal. Using collaborative authoring methods collates feedback from multiple parties in one document, allowing medical writers to make the necessary revisions efficiently. |
Medical writing and scientific publishing continues to evolve in response to changing patient needs, regulatory requirements, and technological advancements. In 2023, TFS harnessed innovative technologies in authoring, collaboration, and quality control to support our creation of compliant, accurate, and ethical reporting of clinical research.
TFS HealthScience: Your CRO Partner for Medical Writing Services
At TFS, we approach every writing project with confidence, flexibility, and close cooperation to deliver high-quality publications that promote transparency, inclusivity, and accessibility in clinical research and with a high acceptance rate. As a top global mid-sized CRO, our writers leverage their diverse expertise while accessing knowledge and experience across functional teams, including regulatory, safety, data management, and biostatistics, ensuring the accuracy and compliance of each manuscript. With experience in all study phases and key therapeutic areas, our team is proficient in developing different publications for diverse audiences, including physicians, patients, and researchers. We can assist with any medical/scientific communication, tailored to your specific requirements. Entrusting TFS with your communication needs will ensure efficiency, continuity, and comprehensive support in every aspect of communicating your clinical study’s results.
Learn more about our medical writing services: https://tfscro.com/solutions/medical-writing/
Connect with Us
Contact us today to discover how TFS can be your strategic CRO partner in clinical development.


