What is an Oncology CRO?
Oncology contract research organizations (CROs) provide clinical trial management services to pharmaceutical, biotech, and medical device companies (sponsors), working under Good Clinical Practices (GCP) and harmonization guidelines while ensuring research quality. Oncology CROs partner with sponsors from the discovery and clinical development of oncology drugs to manufacturing, commercialization, market access, and beyond. The increasing global prevalence of cancer is a driving force behind the expansion of research and development (R&D) and clinical trials for cancer. In contrast, the rising demand for cost-effective drug development supported by oncology therapeutic expertise continues to fuel demand for Oncology CRO services.
Read on to discover more about oncology CRO services provided to pharmaceutical, biotech, and medical device companies and how they help to advance cancer therapies through clinical trials.
Market for Oncology Treatment
Analysts estimate that the global oncology drugs market will grow at a compound annual growth rate (CAGR) of 11.43% from 2023 to reach $401.31 billion by 2032. The most common cancers include lung, breast, prostate, and colorectal cancer. By drug class type, targeted therapy led with over 50% of the cancer drugs market, followed by immunotherapy (24%), chemotherapy (21%), and hormonal therapy (7%). Oncological clinical research and development of oncology drugs is challenging as researchers must contend with regulatory requirements specific to cancer treatment modalities, and before that, the complexity of innovative trial designs and difficulties in recruiting sites and patients, amongst others.
Cancer/Oncology Drug Market Size, 2023 to 2032 (USD billion)
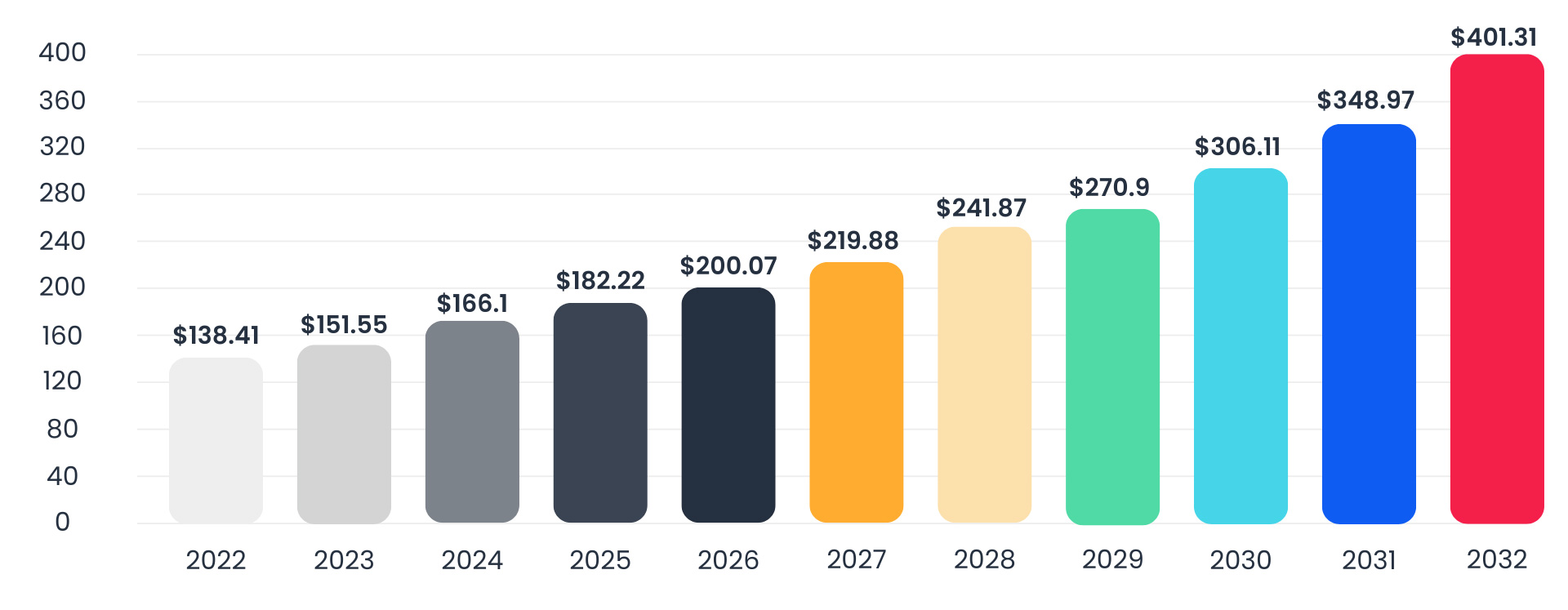
Source: www.precedenceresearch.com
What is an Oncology CRO?
The rising demand for cost-effective drug development with oncology therapeutic expertise increases the demand for Oncology CRO services as outsourcing R&D also enables sponsors to optimize internal cost structures. A contract research organization (CRO) is a specialized provider outsourced on a contractual basis by pharmaceutical, biotech, and medical device companies (sponsors) to manage various aspects of clinical trials. Oncology CROs provide clinical trial management services to sponsors, working under guidelines of Good Clinical Practices (GCP) and harmonization while ensuring clinical research quality. CROs also collaborate with other solution providers to develop innovative products that bring value to sponsors, e.g., Premier, Inc.’s PINC AI™ Applied Sciences (PAS) recently announced a partnership with TFS HealthScience to revolutionize clinical trials by leveraging real-world data and evidence (RWD/RWE) and artificial intelligence (AI).
Oncology CROs’ revenue comes mainly from sponsors’ R&D budgets. As such, future revenue growth for Oncology CROs can be directly related to overall R&D spending and growth in pharma R&D outsourcing.
Demand for Oncology CRO Services
Growth drivers of the global oncology clinical trials market include the increasing global incidence of cancer, increasing investment by sponsors in oncology R&D, growing interest in personalized medicine, a regulatory environment that supports oncology clinical trials, and advancements in tech, e.g., enhanced diagnostic tools and data analysis. Examples of tech transforming cancer research and care include the revolutionary gene-editing tool CRISPR; AI to improve diagnosis, drug development, and precision medicine; telehealth, which brings cancer care and clinical trials to patients; and Cryo-EM, which captures high-resolution images of molecules.
In 2023, the global oncology clinical trials market was $12.98 billion and is estimated to grow at a CAGR of 5.3% from 2024 to reach $21.65 billion in 2033. By study design, interventional studies generated over 88% of revenue in 2023. By geographical region, North America contributed 42% of revenue, and Asia Pacific had the fastest CAGR between 2024 and 2033. The oncology clinical trials market size for the U.S. was estimated at $3.82 billion in 2023, growing at a CAGR of 5.5% to reach $6.49 billion by 2033.
The global oncology clinical trials market dynamics is characterized by rising cancer incidence, which fuels the market, while challenges in patient recruitment and retention restrain market growth. On the upside, the shift to patient-centric trial designs creates opportunities for innovation and efficiency.
Unlocking Success with Oncology CRO Collaborations
Oncology CROs like TFS HealthScience provide oncology and hematology scientific, medical, regulatory, and operational expertise and tailored solutions to ensure the successful execution of complex oncology trials. Sponsors have access to Oncology CRO teams with in-depth therapeutic, scientific, and strategic knowledge and who ensure extreme attention to detail for this highly specialized discipline. Oncology CROs support all phases of development, from the early phase of oncology drug discovery through to Phases II – III (approval), commercialization, market access, and the late phase (real-world evidence (RWE)).
In partnership with Oncology CROs, sponsors benefit from the therapeutic expertise, specialized knowledge, and vast experience of CRO oncology teams at each stage of cancer research. Oncology CROs offer advanced oncology technologies, data analysis expertise and insights generated, real-world intelligence, support in navigating complex regulatory landscapes, and commercialization strategy optimization. In line with global oncology drug market changes, Oncology CROs share their expertise across a growing range of cancer therapies, from immuno-oncology and targeted therapies to novel and emerging therapies, including cell and gene therapies. In addition, sponsors benefit from CROs’ strategic relationships with sites experienced in oncology trials.
Oncology CRO Capabilities and Services
1. Clinical trial design and management
Trial design and delivery, for example, include incorporating patient feedback and insights, leveraging adaptive and novel trial designs, and applying AI and machine learning (ML). Patient surveys and burden analyses inform patient-centric trial designs.
2. Tailored solutions
Oncology CROs offer different models to meet sponsor needs, including full-service CRO solutions, Functional Service Partnership (FSP) solutions, which provide sponsors access to resources to support key functional areas, and Contract Development and Manufacturing Organization (CDMO) services.
3. Site and patient recruitment
Site and patient recruitment and retention strategies and execution, including securing high-performing sites, expediting start-up, using biomarkers for precision oncology trial recruitment, and improving patient recruitment and retention.
4. Digital solutions
Oncology CROs offer digital solutions, including decentralized clinical trial (DCT) solutions, site portals, digital platforms, evidence platforms, and electronic Trial Master Files (eTMF).
5. Cancer modalities
Oncology CRO services extend to specific cancer modalities, including targeted therapies; immuno-oncology, which involves an understanding of its complex challenges and considerations as related to operational issues, biomarkers, safety, and imaging; precision oncology, providing knowledge of the science and technology, operational and regulatory needs, and data analysis requirements to advance precision oncology; radiation oncology and radiopharmaceuticals clinical research; and novel and emerging therapies such as cell and gene therapies.
6. Imaging and laboratory services
Medical imaging and in-house laboratory capabilities, e.g., integrated tumor imaging services.
TFS HealthScience – Accelerating Market Approvals for Life-saving Compounds
Sponsors partnering with TFS Oncology and Hematology CRO gain access to our global, international, and regional experience in oncology, including our global key opinion leaders and site network. In addition to in-depth therapeutic, scientific, and strategic knowledge, TFS ensures operational delivery excellence through proactive planning, risk mitigation, and standardized processes. Our partnership-minded approach and customer-centric focus drive us, honed every step of the way by the support and oversight from TFS’ most senior personnel.
The TFS Oncology and Hematology CRO has supported more than 300 clinical trials in oncology and hematology across all phases. Our experience over the last five years includes indications such as non-small cell lung cancer (NSCLC), breast cancer, prostate cancer, solid tumors, human papillomavirus (HPV)/cervical cancer, and malignant hematology studies. From complex early-phase trials, including basket trials, precision medicines, immuno-oncology, and rare hematologic diseases, the TFS team is here to support the development of your trial. Connect with a TFS representative today!
Learn more about our related services and resources:
Contact Us:
Contact us today to learn more.
