Top 5 Early Signs of Melanoma
Melanoma is the most serious form of skin cancer. According to the U.S. Food and Drug Administration (FDA), melanomas represent approximately 1% of all skin cancers. However, they account for a significant number of cancer-related deaths. The global burden of melanoma is projected to increase despite recent advancements in treatment and many melanoma cases being preventable. Newly diagnosed cases are estimated to increase by more than 50% by 2040 (compared to 2020), and deaths attributed to melanoma are estimated to increase by 68% over the same period. In 1995, the American Academy of Dermatology began Melanoma Monday, which occurs every first Monday in May, designated as Skin Cancer Awareness Month.
Melanoma Monday 2024 is on May 6 and is dedicated to raising melanoma awareness by sharing insights from board-certified dermatologists, key facts and evidence related to melanoma prevention, and personal stories. TFS HealthScience is pleased to contribute to these efforts to motivate and mobilize everyone to take charge of their skin health.
What is Melanoma?
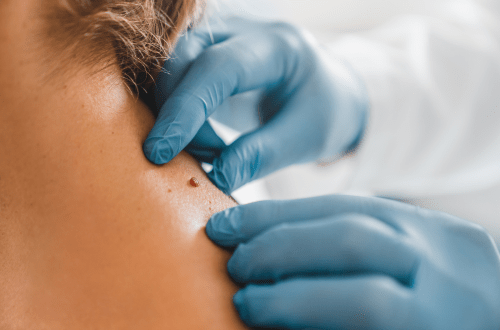
Melanoma, one of three main types of skin cancer, is often caused by exposure to ultraviolet (UV) light from the sun, tanning beds, or sunlamps. However, it can also develop in areas not exposed to the sun. If not detected early and treated, melanoma can metastasize to other parts of the body. Melanoma starts in the pigment-producing cells (melanocytes) and usually affects adults but may also occur in youth. Melanoma appears as a flat or slightly elevated brown lesion with variegation (black, blue, pink, or white discoloration), an irregular shape, and larger than 6mm. Melanoma may begin as an existing mole or on otherwise healthy skin. Symptoms can happen anywhere on the body and also inside the body.
The Melanoma ABCDEs of Self-Exams
The National Cancer Institute (NCI) advises that screening for skin cancer can be conducted by the patient through a visual self-exam and the healthcare provider through a clinical examination. Keeping in mind that melanomas come in different forms and may not display the typical early signs, the first signs and symptoms often are a change in an existing mole, a sore that won’t heal, or a new pigmented or unusual-looking growth on the skin.
The five early signs of malignant melanoma can be remembered as the A-B-C-D-Es of melanoma below.
1. Asymmetrical
Melanomas may look like asymmetrical growths, often larger than 6mm, with irregular borders and variegated color. Unlike common moles, melanomas are typically asymmetrical, and drawing a line down the middle of the suspected growth reveals two halves that don’t match.
2. Border irregularity
While common moles tend to have smoother, even borders, melanomas tend to be irregular or jagged. Melanoma borders may be scalloped, poorly defined, or have notched edges.
3. Color variation
Variegated pigmentation, i.e., varying colors of black, blue, pink, or white from one area of the spot to the next, is a warning sign. Benign moles tend to be a single shade of brown. Melanoma may start with shades of brown, tan, or black, and red, white, or blue may appear as it grows.
4. Diameter
When diagnosed, melanoma is usually larger than 6mm in diameter but could be smaller. If a mole or spot is 6mm or larger, it is a warning sign, especially if it has other ABCE features.
5. Evolving
The last early sign to look out for is if the mole or spot has changed in the previous few weeks or months.
The Centers for Disease Control and Prevention (CDC) advises checking our skin regularly to help detect suspicious changes, not forgetting less visible areas such as the soles of our feet. If you notice the early signs of melanoma, seek advice from a doctor and describe any unusual spots or moles or observed changes. In your assessment, your doctor will ask about your personal and family health history, conduct a physical exam, and perform other tests, including a skin exam and biopsies.
Current Treatment Options
Treatment options for melanoma depend on its thickness, the part(s) of the body it affects, the speed with which the cancer cells are dividing, whether there is bleeding or ulceration, how much cancer is in the lymph nodes, and the patient’s general health. Additionally, the treatment choice considers the lactate dehydrogenase level in the patient’s blood and whether the cancer has specific mutations.
Current treatment for patients with melanoma includes surgery, chemotherapy, radiation therapy, immunotherapy, and targeted therapy, which work by targeting a specific genetic or molecular feature of abnormal molecular pathophysiology. Effective systemic therapy for melanoma includes two classes of treatment: immune checkpoint inhibitors (ICIs) and small molecule BRAF/MEK inhibitor therapy. Combination regimens, e.g., ICIs with anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) agents, have received regulatory approval for some types of melanoma, with long-term follow-up data suggesting that cure is possible for some patients with advanced melanoma. In February 2024, the U.S. Food And Drug Administration (FDA) approved the first cellular therapy to treat unresectable or metastatic melanoma.
Advanced melanoma
Treatment options for advanced melanoma have progressed markedly in recent years, and the condition, which was almost always fatal in the past, now has a five-year survival rate of approximately 50%. Immunotherapy and targeted therapy have progressed, with ICIs making the most significant impact on advanced melanoma13.
Unresectable or metastatic melanoma
Treatment for patients with unresectable or metastatic melanoma may include immunotherapy using PD-1 inhibitors and drugs targeting the BRAF gene for melanoma associated with BRAF gene mutations. For some patients, the melanoma continues to progress after receiving these therapies. The FDA-approved cellular therapy provides a new treatment option for this patient population.
Clinical Trials Focused on Melanoma: Highlights
Although significant progress has been made in melanoma therapy in recent years, many important clinical questions remain unanswered. New treatment options for melanoma are being studied in clinical trials, including developing novel immunotherapies, such as checkpoint inhibitors and agonists, cytokine therapies, oncolytic viruses, and tumor-infiltrating lymphocytes (TILs).
New drug development
For patients with metastatic melanoma who have received previous treatments, clinical research is ongoing to develop new compounds as monotherapy or in combination with existing drugs. Other studies include, e.g., new drug development for patients with metastatic uveal melanoma and other GNAQ/11 mutant melanomas and a clinical study assessing the safety, tolerability, pharmacokinetics, and antitumor activity of a new compound in patients with advanced melanoma with and without current treatment.
New combinations
New oncolytic virus combinations for melanoma have demonstrated encouraging preliminary results. Clinical trials on immunotherapy combinations, e.g., a clinical study on a novel immunotherapy combination in the neoadjuvant setting for patients with stage III melanoma and a high risk of recurrence, are ongoing.
Adoptive cell therapy
There is ongoing research in adoptive cell therapy, a type of immunotherapy where the immune cells within the patient’s tumor, or TILs, are isolated and modified in a lab to be better cancer-fighting cells.
Real-world data
Studies using real-world data investigate the clinical management, treatment patterns, and outcomes of advanced melanoma patients receiving approved first-line metastatic treatments in both adjuvant and unresectable settings.
TFS HealthScience and Dermatology Research
TFS HealthScience is a global contract research organization (CRO) that supports biotechnology and pharmaceutical companies throughout their clinical development journey. TFS demonstrates scientific and medical competence across populations and therapeutics, including industry-leading capabilities in oncology, as well as dermatology, immunology, and inflammatory diseases. It has conducted hundreds of studies in over 30 countries in these therapeutic areas.
Our team specializes in managing clinical development Phases I-IV, RWE studies, and patient recruitment across multiple indications. With our in-house experts, TFS has a global network of more than 8,000 sites and nearly 81,000 patients for our dermatology, immunology, and inflammatory disease trials, as well as oncology and hematology. We partner with you to develop a regulatory and operational strategy tailored to your specific trial’s requirements, then deliver all the scientific and medical aspects of your clinical program to help ensure market success.
Download our one-pager on our dermatology expertise here!
Download our one-pager on our oncology expertise here!
Want to learn more? Contact a TFS team member today!
Connect with Us
Contact us today to discover how TFS can be your strategic CRO partner in clinical development.